Current treatment methods for patients with gliomas are hampered by a poor understanding of underlying biology. Glial cells are critical for brain metabolism, neuronal protection, and cell-cell communication. As a group with long-term experience in studying the function and development of astrocytes and NG2 glia, we are interested in how gliomas interact with adjacent normal glial cells and how glial cells create a microenvironment that influences glioma cell survival, proliferation, and invasion.
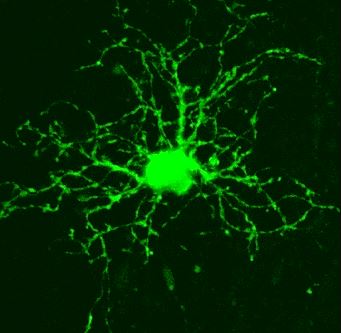
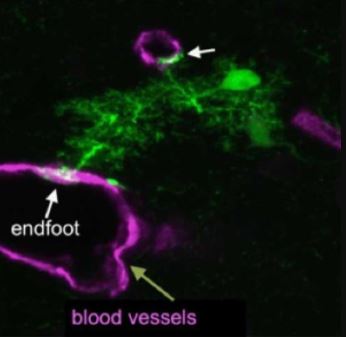
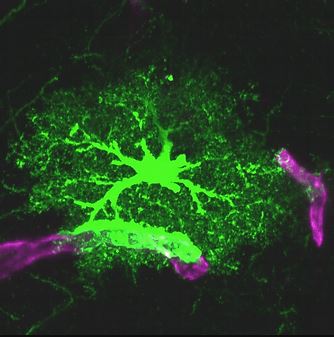
NG2 glial cell An immature astrocyte Astrocyte & its endfeet
Glia-Glioma Interactions
The brain consists of multiple cell types that form a complex neuron-glia-blood vasculature network. During glioma (brain tumor) development, tumor cells infiltrate normal brain tissue and interact with adjacent stromal cells in this network . The network provides glioma cells with an appropriate environment for colonization, growth and infiltration. However, the role of normal glial cells, which constitute 50 percent of cells in the human brain and are critical for a number of functions (brain metabolism, neuronal protection and cell-cell communication), in glioma progression is poorly understood. Improving our understanding of glia-glioma interactions and discovering the underlying mechanisms are critical for the diagnosis, prognosis and treatment of pediatric glioma and necessary to identify new therapeutic targets.
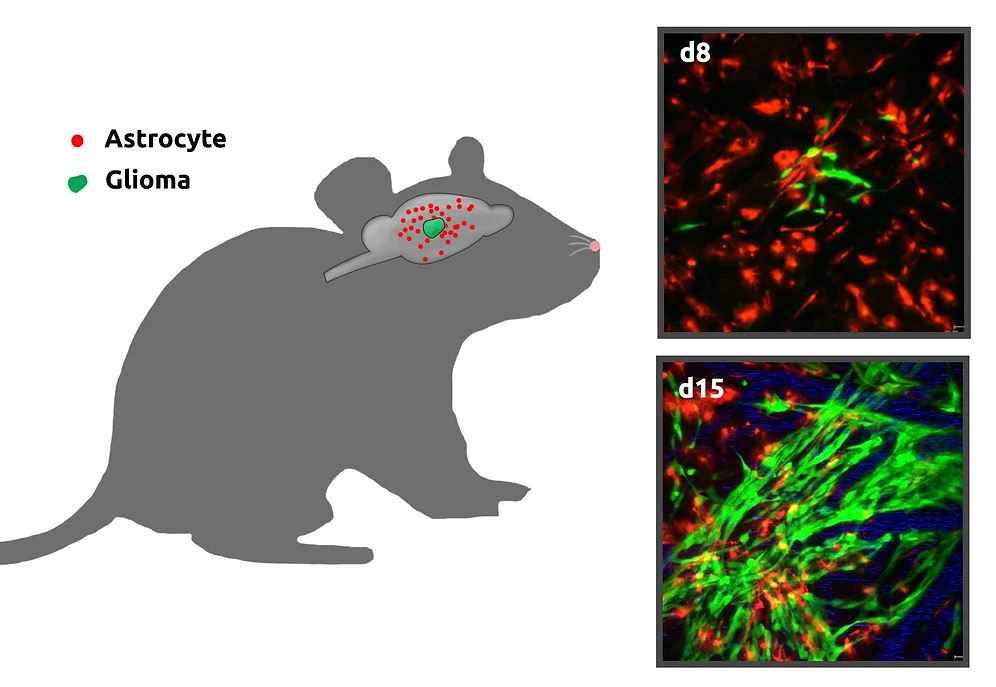
In vivo imaging of growth of gliomas in mouse brains
We are currently investigating if and how different types of glial cells, especially astrocytes, create a microenvironment that promotes glioma cell survival, proliferation and invasion. This includes characterization of the fate and potential functional alterations of astrocytes adjacent to gliomas in vivo. We will perform longitudinal time-lapse imaging to characterize astrocyte properties (survival, proliferation and progeny) within and close to gliomas at different developmental stages. This will provide researchers with a functional paradigm for glioma growth from its initiation through the late stage.
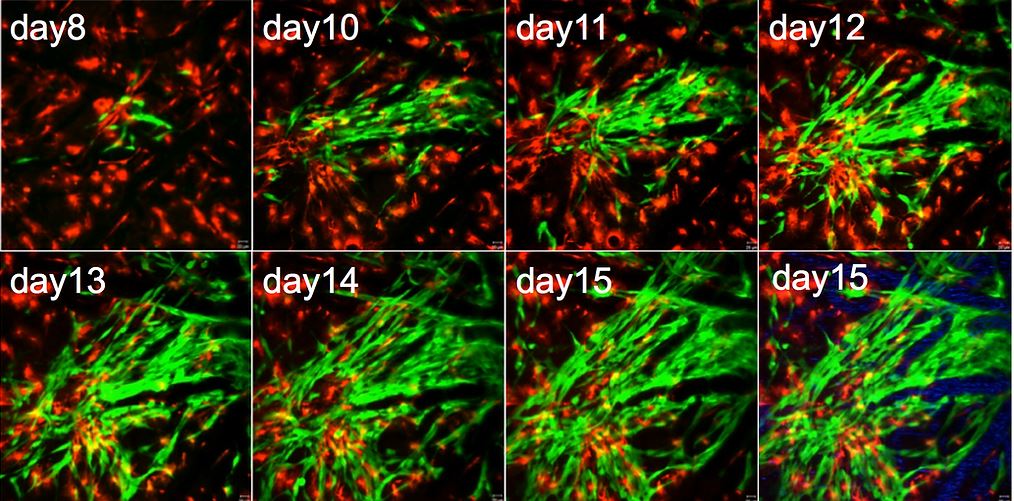
Time-lapse imaging of growing gliomas in mouse brain (green, glioma cells; read, astrocytes)